
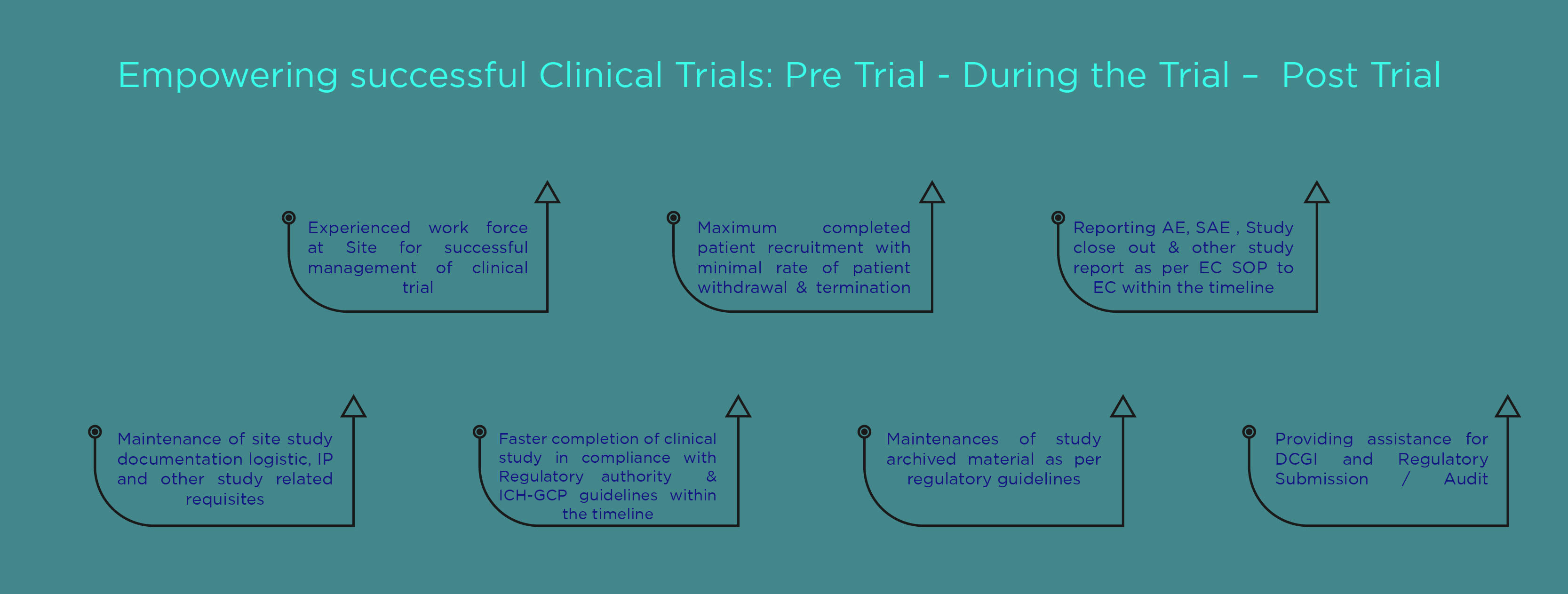
Site Monitoring Operations
Our Site Monitoring Operations has developed one of the largest networks of investigators and investigative sites and maintain high retention of our clients due to our commitment, responsiveness, flexibility, performance. The investigators and sites undergo robust feasibility survey process experience ‘on target’ completion and lesser delays than those who do not undertake such assessments.
The Study Feasibility
Conducting Robust Site Feasibilities: An Investment to Ensure a Good Clinical Study
NUCLEON SITE SELECTION CRITERIA
- Ethics Committee Registered Site by CDSCO with maximum patient pool
- Timer Period of EC meeting to fasten-up EC approval for Clinical Trial
- Cost effective EC meeting Fees
- Experienced DCGI audit qualified Clinical Trial site
- Well Equipped Infrastructure with Clinical Trial Specific requisite which includes Experienced Clinical Trial Team, Logistic, Clinical Trial Material and IP storage unit, NABL accredited Lab / Out-sourcing Lab Vendor Tie-up, and Archival Unit.
The study feasibility is an integral and critical initial step in the conduct of any clinical trial efficiently. By assessing potential investigators at the site level, we have a more accurate analysis of their workload and competing studies. Our proven comprehensive site support model involves placing a full-time, highly qualified clinical research coordinator at each study site to assist the investigators and site staff with all day-to-day study conduct activities.
We help our Doctors transform into successful Investigators
Our Investigators are recruited based on their history of patient recruitment, data quality, and feasibility report based on the specific protocol.
Investigator Selection Criteria:
- ICH-GCP trained
- Experience in Clinical Trial
- Patient Pool
- Completing patient recruitment within the timeline
- Conducts Clinical Trial within Sponsors Budget.
Our Network
- 100 multispecialty hospital networks across PAN India.
- 300 investigators & investigative sites located in more than 25 cities PAN India.
- Investigative site supported by a full-time study coordinator.
- Over 150 data points are collected &evaluated for each site
- Eight Therapeutic Areas
- Our most of the sites experienced successful US FDA & EMA inspections
Patient support programs
Accelerating Recruitment & Retention Strategy in Clinical Trialsthrough Patient Support Programs (PSPs) 24×7 inbound Medical Information Call center (MICC). PSPs are an ideal way for you to directly support patients, improve adherence and optimize efficacy and outcomes for Clinical Trials.
Empowering Effective Patient Recruitment & Retention Strategy in Clinical Trials through PSP
Five key needs that our comprehensive patient support programs address.
Clinical: Providing patients with a point of contact during trials and assisting with transitioning from clinical to commercial drugs, specifically clinical trial support and risk evaluation and mitigation strategies.
Engagement: Specific engagement related solutions include enrollment and consent, case management, patient adherence programs, portals, assistance with appointments and scheduling, mobile health monitoring, telehealth, and advocacy.
Therapy: Connecting with patients to provide access to and support with their care. Specific therapy-related solutions include access to care, specialty pharmacy triage, distribution solutions, site of care/infusion site match, lab/test results coordination, and nurse visits.
Financial: Specific financial solutions include insurance verification, benefits investigation, claims appeals and re-coding, prior authorization, co-pay assistance, and bridge therapy programs.
Education: Solutions include medical information and pharmacovigilance, nursing educational support, and between-visit care.
- 24x7 Inbound support line for advice
- Access to registered nurses, clinicians and pharmacists
- Site training material for patients, customized to geographical and regulatory compliances
- Exhaustive monitoring with focus on protocol requirements to better identify
- Risk, non-compliance and poor performance and timely reporting of adverse events
- Planning for patient-visit schedules according to study parameters
- Reminder support alerts for missed appointments and medication
- Analysis of data trends including enrollment/dropout status and adverse events
- Fitness, diet counseling, smoking cessation and alcohol reduction programs
- Caregiver support as per criticality/complexity of disease in the trial
